Rheumatoid arthritis (RA), is a systmic chronic autoimmune inflammatory disease of the joints resulting in cartilage and joint destruction. RA affects 0,5-1% of the population with a onset typically between ages 25-50 and a predominance in women. Disease pathogenesis is highly heterogenous and influenced of both environmental and genetic factors. Also disease devleopment and disease course is hetergenous. The clinical phase of disease when symphtoms appear and also treatment is initiated is proceeded by a preclinical where the immune system build up a response towards the target organ. RA is characterized by inflammation in the synovial membrane, leucocyte infiltration, joint swelling and pain. Although joints are the main target of the disase progress in RA, patients may present with extra-articular features including sub-cutaneous nodules, vasculitis and pulmonary fibrosis. In addition, there is a systemic inflammatory response with eleveated levels of autoantibodies and acute phase proteins.
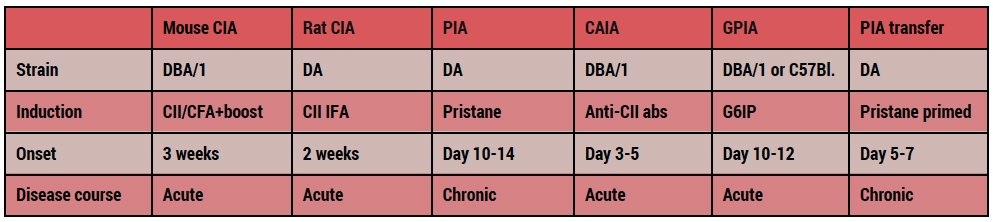
Due to this complexity of the disease, a singe in vivo model cannot mimic RA there are a number of valid models reflecting different aspects of the human disease. Redoxis offers passively and actively induced models of RA in both rats and mice. All have their own chararcteristics and cellular mechansims and carefull model selection is therefor crucial for sucsessfull experiments.
Rat and mouse models mimicking different aspects of the pathogenesis in human disease are invaluable tools to understand the basic biological mechansms, to identify and validate novel disease mediating pathways and targets and to screen and evaluate efficacy fo potential preventive and therapeutic agents. All models exhibit classical features fo RA including joint swelling, synovitis, pannus formation and bone erosion but differ in onset, chronicity, severity, resolution and histopathology.